The term “wetting” is commonly used to describe the flow of braze alloy or braze filler across a surface via capillary action; it is critical for a successful braze joint. However, before we get more into wetting, let’s recap the brazing process.
Basic Furnace Brazing Process
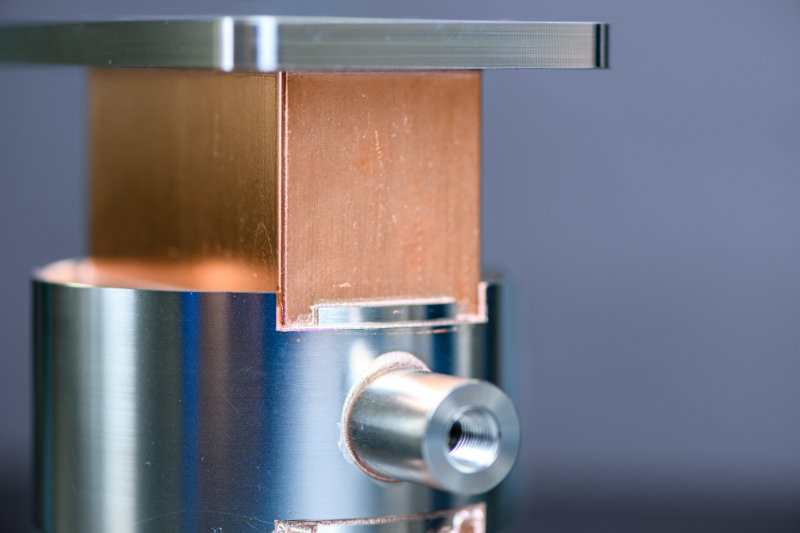
First, two components are placed very close to or in contact with each other and a braze filler metal is placed in contact with both parts. The filler and components are heated to just past the melting temperature of the filler braze alloy. The filler alloy “wets” the parts and capillary forces draw the braze alloy between the mating components, making up what is called the braze joint. The assembly is then cooled, solidifying the braze joint.
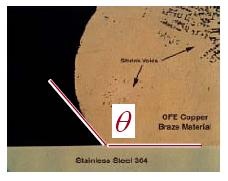
Brazing is an art, a science and very sensitive to proper joint design, material preparation, and wetting. Our brazing services always provide a deep dive review from our engineering team to ensure a successful braze. Good wetting occurs when the surface is clean & metallic and is measured by a Contact Angle:
Classical Wetting Braze Flow
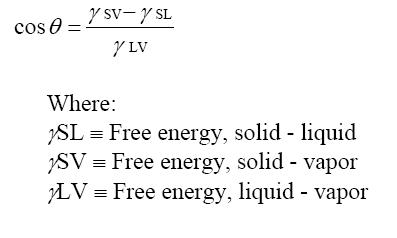
Classical wetting has no chemical or metallurgical interaction and this system can almost be achieved by a filler/substrate combination of Cu on Mo, where the solubility of Cu in Mo is minute. Classical wetting braze flow rarely happens and in most systems, there is substantial solubility or formation of intermetallics whereby metallurgical and chemical interaction forces are dominant.
The forces of chemical interaction are exploited with active metals such as titanium, and these types of active metals can be used to wet ceramics. Here at Altair Technologies we routinely perform active metal brazing, achieving hermetic bonds between metals and ceramics.
Contact Angle & Surface Roughness
If the contact angle is less than 90º, for two clean and relatively smooth surfaces, the surface energies will result in capillary attraction, which then experiences laminar flow wetting. Flow velocity increases when 1) surface tension increases, 2) when the joint gap increases, 3) when the contact angle decreases and when 4) viscosity decreases.
Surface roughness reduces the effective contact angle causing more spreading and scratch marks or “lines” act as capillaries causing directional spreading in one direction but also act as dams reducing the spread or flow of alloy in the perpendicular direction.
Good Wetting Poor Wetting Great Wetting
Eutectic and fundamental fillers have low viscosity spreading while non-eutectic fillers have a higher viscosity, especially in the “mush” zone. Any alloy segregation while melting (liquation) will experience less spreading. Increasing temperature above the melting point decreases viscosity and extends the molten alloy range giving more spreading. It is important to understand that filler/substrate interaction can either facilitate or impede spreading.
In the case of Cusil alloy on Copper, where erosion produces excessive spreading, it is important to control temperature otherwise the elevated temperature decreases viscosity and the dissolution increases the volume of melt.
The opposite is true in the case of Copper (used here as a filler alloy) on Nickel, where erosion produces poor spreading and the increasing temperature does not improve flow much, because although the elevated temperature reduces viscosity, the dissolution increases, which counters by raising the viscosity.
Why or when does filler/substrate interaction produce poor spreading in some cases and excessive spreading in others? By examining phase diagrams we can explain the following observations:
- Cusil on Cu blushes (excessive flow)
- Cu/Au on Cu flows well
- Cu on Ni flows poorly
- Cu on Fe flows very well (blush potential)
Contact one of our advanced material joining specialists today for a quick feasibility study of your project.