High Vacuum (UHV) & High-Temperature Applications
Copper is a vacuum compatible material with excellent electrical and thermal conductivity properties. It is used in many UHV, high-vacuum, and high-temperature applications as well as in virtually all RF power, ion beam, and electron beam devices such as linear accelerators, x-ray tubes and traveling wave tubes. It is also one of the most commonly brazed metals our customers purchase from our Brazing Services here at Altair Technologies. While we do perform vacuum brazing with a variety of braze alloys, we primarily use Hydrogen brazing because it is arguably the best process for bonding metals and ceramics regardless of the application.
Furthermore, we have in-house UHV (Ultra-High Vacuum) bakeout capabilities used as a pre-processing step to better serve all of our UHV, LINAC, and RF component customers.
Furnace brazing in hydrogen gas is the best method to braze copper to itself or to other metals like stainless steel for accuracy, joint-strength, and high-vacuum applications. It always results in a clean and beautiful part because the hydrogen gas helps reduce oxides, removes hydrocarbon contamination and improves the capillary action (wicking) of the filler into the joint. The method of solder brazing or torch brazing uses a solder filler material via a brazing rod for industrial/commercial applications and is the most rudimentary and contaminated method.
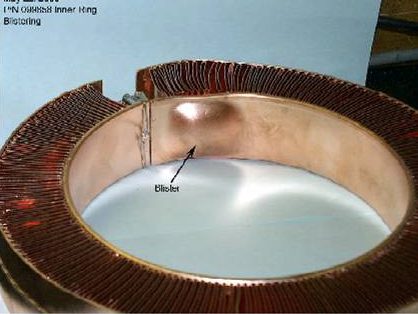
Hydrogen Brazing requires special vacuum grade OFE Copper 101 to prevent the formation of water vapor in the hydrogen furnace that results in bulging or blistering.
The Altair Furnace Brazing Process
Here at Altair Technologies most of our products are for high vacuum applications and we, therefore, do most of our brazing in Hydrogen Gas or in a high vacuum atmosphere. During the braze run, while assemblies are being heated up to braze-temperature, the furnace “bell” sees a steady flow of Hydrogen gas. This process continues until parts are below a certain temperature at which time the bell is backfilled with Nitrogen gas. Products or assemblies that are commonly hydrogen brazed include X-Ray Tubes, Traveling Wave Tubes, Linear Accelerators for medical, research and security applications and similar ion or electron beam devices. For reasons to be explained below, Hydrogen brazing is arguably the best process for bonding metal and ceramics regardless of the application.
Preventing Material Deformation, Blistering & Bulges
Perhaps the most important aspect or lesson to be learned from this post is to use special vacuum grade OFE Copper 101 as explained above to mitigate the possibility of blistering, bulges or rough surfaces.
We’ve learned over the years that not all OFE copper is equal and more importantly not all suppliers are reputable. Here at Altair Technologies, we source direct all of our materials to be used in a high vacuum application. When Non-OFE material is used, oxide inclusions form water-vapor that create blisters or bulges or rough surfaces and the braze alloy/filler disappears into opened grain boundaries as shown below. In this scenario, vacuum leaks are inevitable and it should be noted that gas leaks are easily detected but can be hard or impossible to pin-point.
Bulge or blister Seen on Non-OFE Copper Copper Inclusion Grain Boundary Disruption
Mitigating Contamination and Oxides with Hydrogen
Hydrogen (H2) gas acts as a fluxing agent, reducing native oxides and removing hydrocarbon contamination producing an ultra-clean raw metal surface. Many oxides, like iron oxide and copper oxide, are easily reduced by H2, whereas many others like aluminum, beryllium, titanium, and silicon can be very tenacious and will not braze or reduce properly in wet or dry H2.
The decision to braze in “wet” or “dry” Hydrogen can depend on a few important aspects such as the base or substrate materials being used and/or the filler alloy type, as well as the application or performance requirements. In cases where the removal of oxides is predominantly important or necessary, dry Hydrogen is used and if the user is more concerned with the removal of hydrocarbon contaminants, it is advisable to braze in wet Hydrogen.

Copper is usually brazed in wet hydrogen, however, depending on the other materials being brazed to it, like stainless steel where the removal of oxides is necessary, dry hydrogen can be used. Here, we have a reactive family of elements that may form undesirable compounds and are therefore typically brazed in high vacuum or with other inert gases like helium or argon.
Chromium (Cr), which is a large constituent of Stainless Steel (SST), occurs near the middle of the oxidation/reduction equilibrium space that hydrogen furnaces can produce. As desired, we can form chromium oxide or reduce that oxide by the atmosphere’s dew-point for temperatures greater than 800 ºC. For chromium-rich stainless steel (SST), we braze in a dry hydrogen atmosphere or if plated with nickel or another suitable metal, we can alternately braze in a wet hydrogen atmosphere.
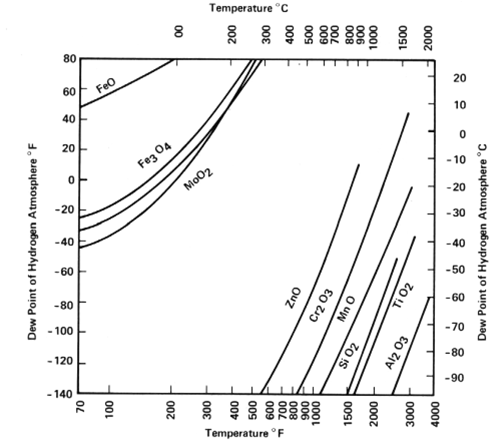
The chart below shows the temperature and/or dew point where the native oxides for various metals can be reduced. FYI, dew points below -60 C are not achievable.
Cr2O3 stable even in Dry H2
Braze-Joint Strength & Requirements
Proper design of braze-joints, surface finishes, flatness and more are paramount to ensuring a strong brazing joint via excellent capillary action to provide wetting and braze flow. Please read our article on wetting, braze flow and filler spreading with regards to braze-joint design, material prep and strength. When brazed-joint design and material preparation are both excellent, then the joint itself will be stronger than the base material.
Hydrogen Furnace Brazing of Copper Brazing Copper to Stainless